21 October 2023 | Healthcare
How New Standards May Shape the Future of Digital Health
By workweek
While digital health funding has slowed down since the height of the pandemic, billions of dollars continue to pour into the digital health space.
But the question remains: are digital health solutions actually solutions?
In this article, I’ll quickly recap the efficacy of digital health technologies, dive into the Peterson Health Technology Institute’s latest framework for evaluating digital health technology, and provide some thoughts on why PHTI may be setting a new (higher) standard for digital health technology.
Are Digital Health Solutions Actually Solutions?
A cross-sectional observational study by Rock Health found many digital health companies lack clinical robustness. The researchers analyzed 200+ digital health companies across different clinical domains (prevention, diagnosing, treatment) and assigned a “clinical robustness score” based on the sum of clinical trials and regulatory filings (510(k), De Novo, premarket approval). Around 60% of the companies analyzed had a clinical robustness score of 1 or less (the median score was 1).
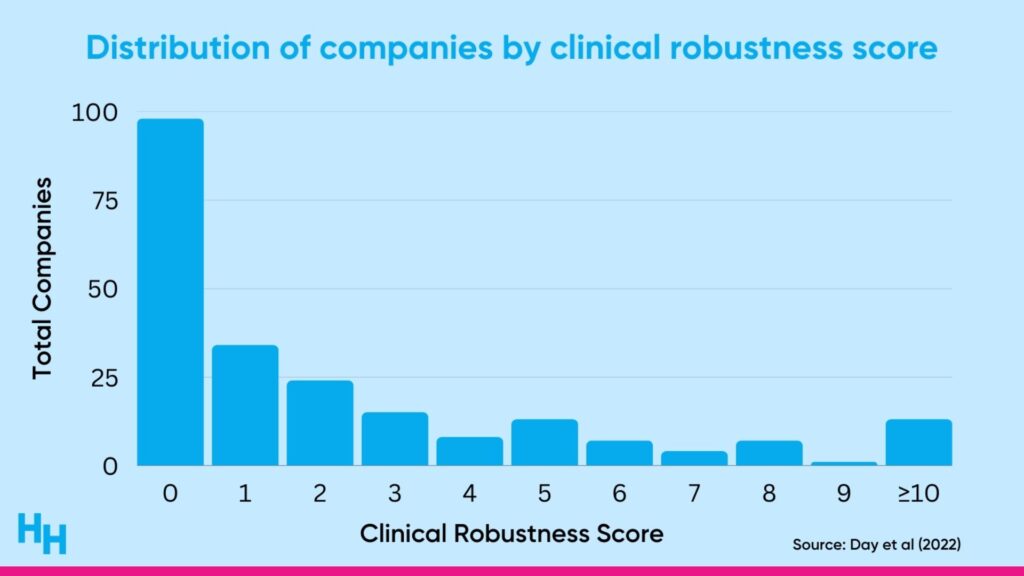
Digital health companies should be validating their solutions and purchasers should be looking for such validation to ensure they’ll receive a positive return on investment. But up until now, there hasn’t been a defined structure—whether that be an institution or framework—to help determine or guide the digital health industry into validating solutions.
The Peterson Health Technology Institute (PHTI) is a newly launched organization that will independently evaluate the clinical effectiveness and economic impact of digital health solutions. PHTI will likely benefit both builders and purchasers:
- Builders are trying to establish themselves among thousands of digital health solutions all vying for purchasers’ attention.
- Purchasers are trying to evaluate which of these thousands of digital health companies will provide the greatest clinical and economic impact—this has been challenging given the lack of tools for such evaluations.
So, just how will PHTI evaluate digital health technology?
PHTI’s New Formula for Grading Digital Health Technology
PHTI’s framework for evaluating digital health technologies revolves around three principle questions followed by a final recommendation:
- What is the digital health technology?
- Does it work and for whom does it work?
- Is it worth it?
What is the digital health technology?
PHTI will first work to understand the “big picture” of the digital health technology to put the remaining part of the evaluation into context. This may include evaluating the following:
- History of digital health technology.
- Market map
- Privacy and security features
- Funding history
In a sense, trying to understand a digital health technology’s story helps level the playing field when it comes time for evaluations and comparisons. For example, digital health technology X that’s two years old from a company with $50 million in funding has to be viewed differently than digital health technology Y that’s from a seven-year-old company valued at $1 billion.
Does it work and for whom does it work?
This question takes into account three different areas, including clinical safety and effectiveness, user experience, and health equity.
Clinical safety and effectiveness: PHTI will evaluate digital health technology based on the evidence supporting its use and its risk to patients. If you recall from my previous article on this topic, the more risky the technology, the more complex the technology likely needs to be, and the more evidence is needed to support its use.
Similarly, PHTI will look at the absolute minimum evidence needed to assess the clinical effectiveness of a digital health technology based on the end-user risk. For example, PHTI would require simple fitness trackers like Fitbit to prove (at a minimum!) it provides valid, accurate, up to date information. However, for something like prescribed behavior change technologies (weight-loss, smoking cessation), the company should have (at a minimum!) a quality observational or quasi-experimental studies.
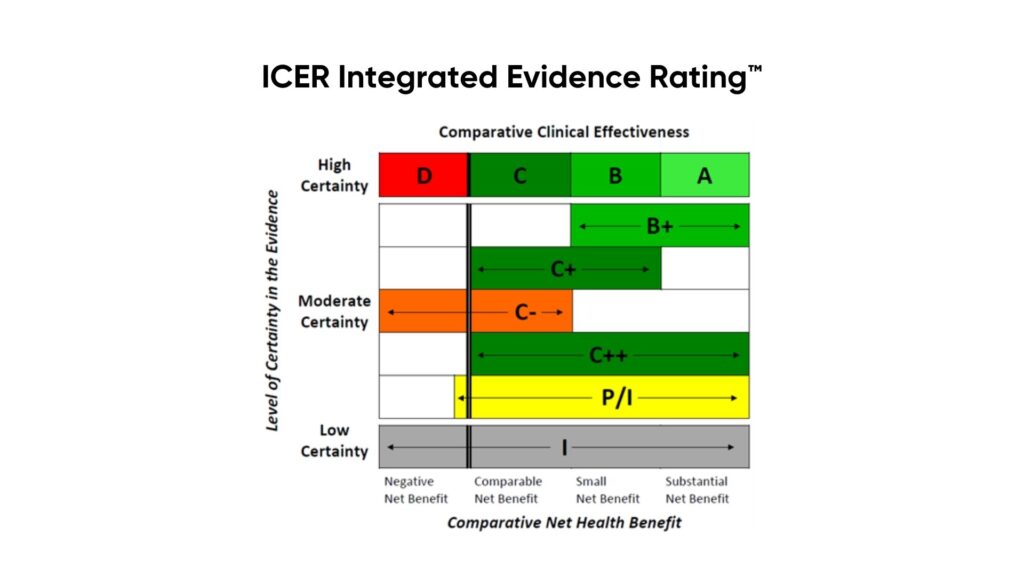
Based on the safety and effectiveness findings, PHTI will use The ICER Evidence Rating Matrix to grade the technology.
User experience: PHTI will evaluate the strengths and limitations of a digital health technology’s user experience based on end-user satisfaction and engagement.
Health Equity: PHTI’s evaluation of equity involves considering digital health technology’s accessibility across diverse subpopulations, their potential in bridging or exacerbating health outcome disparities, and their design and testing to meet the diverse needs of populations.
Is it worth it?
The economic evaluation of digital health technologies will focus on analyzing their budget impact from two perspectives: the individual user, considering out-of-pocket expenses and changes in health management costs, and the payer/plan sponsor, addressing overall healthcare costs and practitioner productivity impacts. This analysis spans a one-year horizon, potentially extending to five years for long-term benefits, and includes varying scenarios and implementation costs.
Tying it all together
PHTI will include all of their quantitative and qualitative data into a single report, with an assigned recommendation essentially saying whether a purchase should adopt or not adopt digital health technology, or if the digital health technology needs further testing.
- Evidence inadequate to support broad field testing
- Evidence adequate to support field testing in broader populations
- Evidence adequate to support wide adoption
Dash’s Dissection
Eventually, digital health technology will be prescribed like medicine in the U.S.
Germany is already setting the precedent.
In Germany, physicians can prescribe approved digital health technology and the health system will cover the costs. The German Digital Healthcare Act (2019) selected digital health technology with proven clinical benefits that will be reimbursed by public payers as digital therapeutics. The government evaluates the digital health technology on clinical evidence, safety, and data protection and security. As of now, 30 digital therapies have been approved.
So, it’s only a matter of time before the U.S. follows suite: but similar to how medications are held to a high evidence standard by the FDA, so too will digital health technology.
This is why I’m quite passionate about PHTI, who will serve as an independent evaluator of digital health technologies. They’re raising the bar. Their framework will hold the digital health space to higher evidence-based standards, which can only be a value-add to the space as it will benefit purchasers, patients, and other users.
In summary, the current landscape of digital health necessitates rigorous evaluation standards, a role that PHTI is actively assuming. Their approach evaluates the effectiveness and economic viability of technologies, ensuring they meet high clinical and equity standards. The adoption of such rigorous standards, as seen in Germany’s healthcare model, forecasts the future of digital health technology in the U.S.
Stay ahead in healthcare with my weekly Healthcare Huddle newsletter, covering digital health, policy, and business trends for 32,000+ professionals. Share with colleagues and subscribe here.