08 October 2022 | Healthcare
Eli Lilly’s To-Be Blockbuster Anti-Obesity Drug Receives Fast-Track Designation
By workweek
The FDA granted Eli Lilly’s revolutionary anti-obesity drug called tirzepatide fast-track designation, expediting the drug’s arrival to market. This is big news for Eli Lilly and the medical community, given clinical trial data shows the drug is extraordinarily effective for weight loss and improvement in cardiometabolic health. I’ve been covering tirzepatide throughout the year (here and here) and truly believe tirzepatide will be the next blockbuster drug.
The Deets
The FDA granted tirzepatide fast-track designation based on promising results from its recent Phase III clinical trial called SURMOUNT-1. The fast-track pathway aims to expedite the development and review of novel drugs to treat serious conditions (e.g., Alzheimer’s and ALS) and fill an unmet medical need.
Tirzepatide is a GLP-1 and GIP receptor agonist that reduces appetite, slows food release from the stomach, increases insulin response and inhibits glucagon.
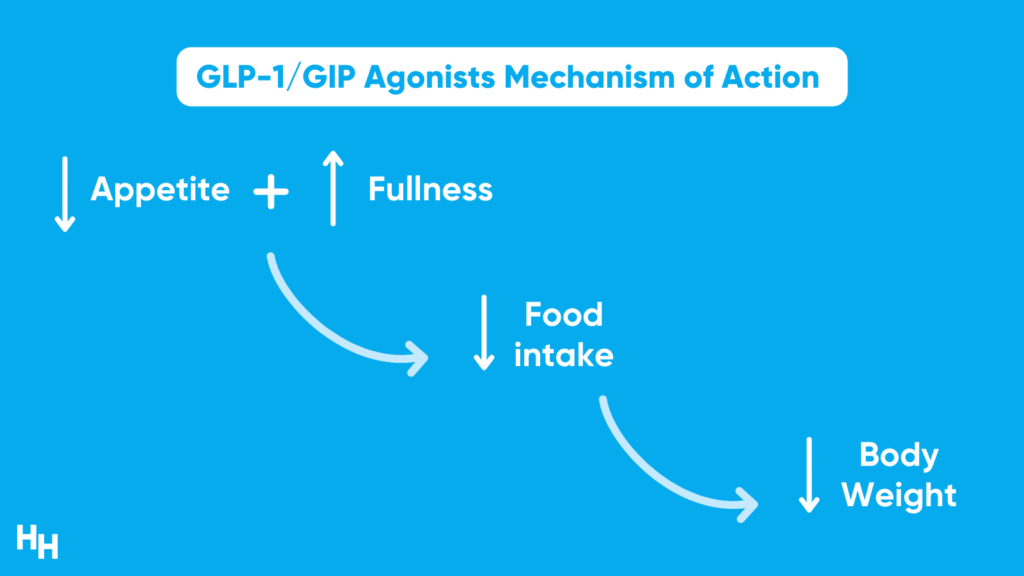
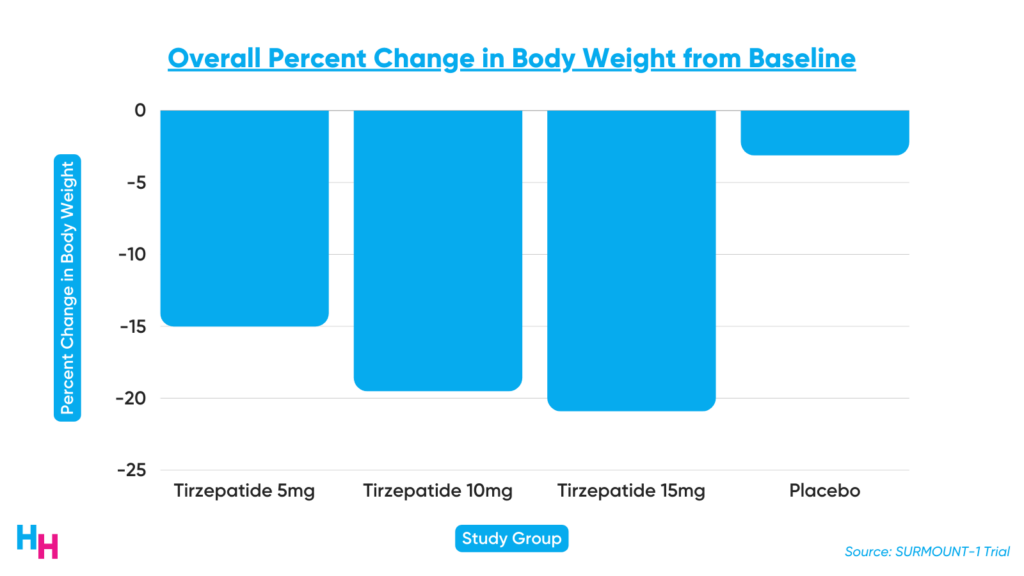
Results from the SURMOUNT-1 clinical trial are promising. SURMOUNT-1 was a 72-week-long Phase III, double-blind, randomized control trial, including 2,500 participants with a BMI over 30, or 27 with more than one weight-related comorbidity that’s not type 2 diabetes. At the end of the 72-week study period, participants’ body weight decreased on average by 16% in the low-dose group, 21% in the medium-dose group and 23% in the high-dose group. Those in the placebo group only lost 2% of their body weight.
Additionally, metabolic health indicators improved in the tirzepatide groups. For example, participants receiving the drug saw a 20% decrease in triglycerides, a 7.5% decrease in non-HDL lipids, and a 39% decrease in fasting insulin (this drug is also an anti-diabetic drug). Also, HDL levels improved by 9% in the tirzepatide groups.
Eli Lilly must complete their SURMOUNT-2 clinical trial before the FDA grants them accelerated approval of tirzepatide. SURMOUNT-2 includes patients with type 2 diabetes (SURMOUNT-1 excluded this population) and will end in the spring of 2023.
Competition
Tirzepatide’s competition is Novo Nordisk’s FDA-approved anti-obesity medication called semaglutide, a GLP-1 analogue. Semaglutide is efficacious, just like tirzepatide, and has been on the market since the summer of last year. The drug raked in $161 million in this year’s second quarter.
Dash’s Dissection
Tirzepatide will be the next blockbuster drug. First, the drug is remarkably effective at inducing weight loss and improving cardiometabolic health with minimal side effects. Second, couple the drug’s superior efficacy with its vast total addressable market (in the U.S., two out of five adults have obesity), and you have yourself a blockbuster drug.
The next decade of anti-obesity treatment will mirror what anti-hypertension treatment was back in the 90s, which became a $30 billion market. Semaglutide and tirzepatide are on track to tap into a global obesity market predicted to be worth more than $50 billion by the end of the decade.
Once tirzepatide is FDA-approved, both tirzepatide and semaglutide will transform metabolic health in modern medicine. The hope is that the medication is distributed equitably across income brackets, races and ethnicities. The fear is that the medications become “privileged” drugs, where only those with private insurance have access to the effective medications while those with public insurance are neglected.
If you enjoyed this recap, share it with colleagues. Sign up for the Healthcare Huddle newsletter here.