01 July 2023 | Healthcare
Medicare vs. Pharma: The Legal Fallout of the Inflation Reduction Act
By workweek
Just as the ball started rolling with the implementation of drug pricing regulations under the Inflation Reduction Act of 2022, pharma and its lobbying organizations promptly jumped in, saying, “Whoa, whoa, whoa, let’s slow down a bit.”
Several drugmakers and lobbying groups have filed lawsuits against CMS and HHS, arguing that the Inflation Reduction Act provision giving Medicare the power to negotiate drug prices is unconstitutional. It just so happens to be the one provision that will greatly benefit Medicare patients but will simultaneously pose a significant threat to drug manufacturers’ revenue streams.
In this article, I’ll highlight the Inflation Reduction Act, dive into who is suing the government, and discuss why drugmakers have every right to be angry.
The Deets
The Inflation Reduction Act, signed in 2022, tackled three healthcare areas: climate change, insurance subsidies, and drug pricing. For the sake of this article, I’ll focus on drug pricing.
The Congressional Budget Office estimates the drug pricing provisions in the Inflation Reduction Act will save the government over $200 billion over a decade. These drug pricing provisions include the following:
- $35 cap on a month’s supply of insulin for Medicare.
- $2,000 annual cap on out-of-pocket prescription drug costs for Medicare Part D.
- Penalties, in the form of rebates to Medicare, for drugmakers who raise their drug prices faster than inflation (first round of penalties here).
- Authority to Medicare (CMS) to negotiate prescription drug prices on selected high-cost drugs: “Medicare Drug Price Negotiation Program.”
Medicare drug pricing negotiation is perhaps the most nuanced of all the drug pricing provisions and will be rolled out over the course of a decade.
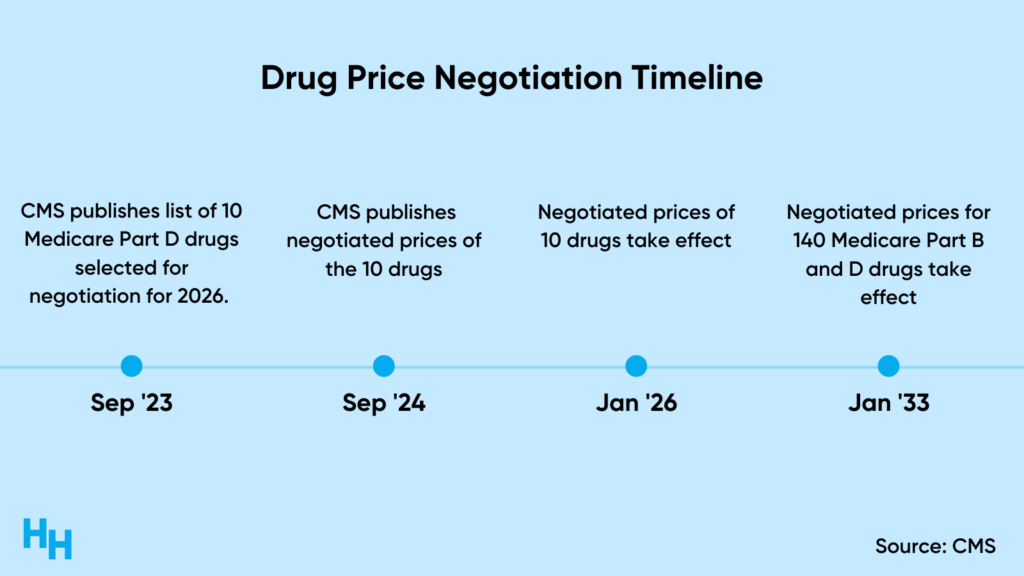
CMS will negotiate the “maximum fair price” for a drug, which is set based on the lower value of data like the average sales price for a Medicare Part B drug or the average price of a Medicare Part D drug weighted for the number of people taking it and discounts received.
Drugmakers will face hefty penalties if they don’t comply with the negotiation process. These penalties include a 65% excise tax on the drug’s revenue in the U.S., which will increase by 10% quarterly to a max of 95%. If drugmakers don’t want to pay the tax, they can withdraw their drug from Medicare and Medicaid formularies. Further, if drugmakers charge patients more than the negotiated price, they’ll pay a penalty equal to 10x the difference between the price charged and the maximum fair price.
Can you see why drugmakers may be angry?
Who is suing the government and why?
The Medicare Drug Price Negotiation Program has angered a few companies and organizations with a lot of money:

These companies and organizations filed separate lawsuits against CMS and HHS, arguing the Medicare Drug Price Negotiation Program is unconstitutional. Specifically, they argue the program violates the 1st, 5th, and 8th amendments of the constitution.
- 1st Amendment Arguments: Merck, Bristol Meyers and Squibb, and US Chambers of Commerce argue the drug price negotiation violates the 1st Amendment by forcing drugmakers to say they “agree” to a negotiated maximum fair price set by CMS. It’s essentially “forced speech.”
- 5th Amendment Arguments: Merck, Bristol Meyers and Squibb, and US Chambers of Commerce argue the drug price negotiation violates the 5th Amendment, claiming the government is taking drugmaker’s private property without just compensation. CMS is essentially obtaining prescription drugs without paying the fair market value.
- 8th Amendment Arguments: PhRMA, NICA, and GCCA argue the program violates the Excessive Fines Clause of the 8th Amendment, saying the penalties for not complying with the negotiations are disproportionate to the offense.
Opponents of the Inflation Reduction Act raise an additional argument, emphasizing the potential stifling of innovation. They caution that implementing price control measures could have adverse effects on drug research and development, thereby posing a threat to future medical breakthroughs. Consequently, they’ve aptly nicknamed the Inflation Reduction Act as the “Innovation Reduction Act.”
Dash’s Dissection
It was obvious from the get-go that patients, physicians, and the government would cheer about the passing of the Inflation Reduction Act while pharma would sit pouting in the corner. This legislation is basically a win for everyone but pharma.
Pharma has every right to sue to try and prevent the Medicare Drug Price Negotiation Program from rolling out. You would be upset, too, if you were about to lose millions in potential revenue. But from a patient or physician perspective, it’s hard to pity pharma. The pharma industry (and drug supply chain, system… whatever you want to call it) has taken advantage of the rules to price their drugs at whatever the market can bear, leaving patients to suffer as they try to afford life-saving medications.
Prior to the Inflation Reduction Act, the pharmaceutical industry had never been held accountable thanks to the provisions of the Medicare Modernization Act of 2003. This act introduced Medicare Part D, but it also included a restriction that prevented the federal government from negotiating drug prices and using value metrics to determine reimbursement rates. The main reason behind this restriction was the concern that it would hinder innovation.
Not surprisingly, opponents of the Inflation Reduction Act are employing the argument of innovation against it, which does hold some merit. There is evidence suggesting that price control measures can decrease revenue, and a 1.0% reduction in revenue can result in approximately 1.5% less spending on R&D. However, considering that pharmaceutical companies are making billions in profits, will price control truly have a significant impact on innovation? Throughout the industry, drug manufacturers have consistently set their drug prices higher than the cost-effective values set by ICER, so price control would reimburse drugmakers for the true value of their drug.
The catastrophic drug affordability problem faced by Americans does not have a simple solution. However, one thing is clear: punishing patients with higher out-of-pocket costs is not the answer. In a thought-provoking NEJM article, the author explores the delicate balance between drug pricing control and fostering innovation. The article proposes several approaches, including value-based reimbursement, increased funding for early-stage research and development by expanding NIH funding, and addressing the costs and risks faced by companies involved in the development of late-stage drugs that have a greater societal impact than financial impact.
It will be interesting to see how these lawsuits play out. I’m no legal expert, so I can’t make any predictions. All I will say is that the VA has a negotiation system in place, and it does a pretty good job at minimizing out-of-pocket prescription drug costs for their veterans. I’ll write about this later on.
In summary, pharma companies and lobbying groups are suing the federal government over the Inflation Reduction Act. They claim it’s unconstitutional, infringes on their rights, and stifles drug innovation by imposing price controls and penalties. Amid concerns about potential impacts on research and development, the Act could also offer significant benefits, including reduced drug costs for Medicare patients. Balancing innovation and affordability in drug pricing remains a complex, evolving issue.
Stay ahead in healthcare with my weekly Healthcare Huddle newsletter, covering digital health, policy, and business trends for 30,000+ health professionals. Share with colleagues and subscribe here.