06 May 2023 | Healthcare
Public Health Emergency’s End Spells Uncertainty for Patients and Digital Mental Health Companies
By workweek
The Covid-19 public health emergency declarations end on May 11th after a three-year run. These declarations provided flexibilities in healthcare policies and rules like continuous Medicaid enrollment, free covid tests, and expanded telehealth access.
Part of the telehealth flexibilities involved the DEA waiving an in-person visit requirement for prescribing controlled substances, allowing patients to obtain medical treatment after seeing a physician or provider via telehealth. This flexibility has benefited millions of patients, especially those with substance use disorder.
So will all those who benefited from the DEA’s waiver, including patients and digital health startups, be out of luck? In this article, I’ll explain the background of the waiver, what happens after May 11th, and how patients and digital health startups will be affected.
Background on the DEA’s Ryan Haight Act Waiver
At the start of the pandemic, the DEA waived the Ryan Haight Act’s in-person exam requirement for prescribing controlled substances, enabling patients to receive needed prescriptions through telehealth.
The Ryan Haight Act, aimed at preventing the illegal distribution and misuse of controlled substances, mandated at least one in-person medical evaluation before prescribing these medications.
Stay-at-home orders during the pandemic increased the risk of patients with opioid use disorder losing access to essential treatments like methadone and buprenorphine (both controlled substances), heightening their risk of overdose. However, before the DEA’s waiver, physicians could not remotely prescribe these medications due to the Ryan Haight Act’s in-person visit requirement, despite the availability of telehealth services.
May 11th, Now What?
In February, the DEA proposed new rules for telehealth prescribing controlled substances after the public health emergency ends on May 11th. These rules are significantly stricter than the relaxed rules imposed throughout the pandemic and involve two critical changes:
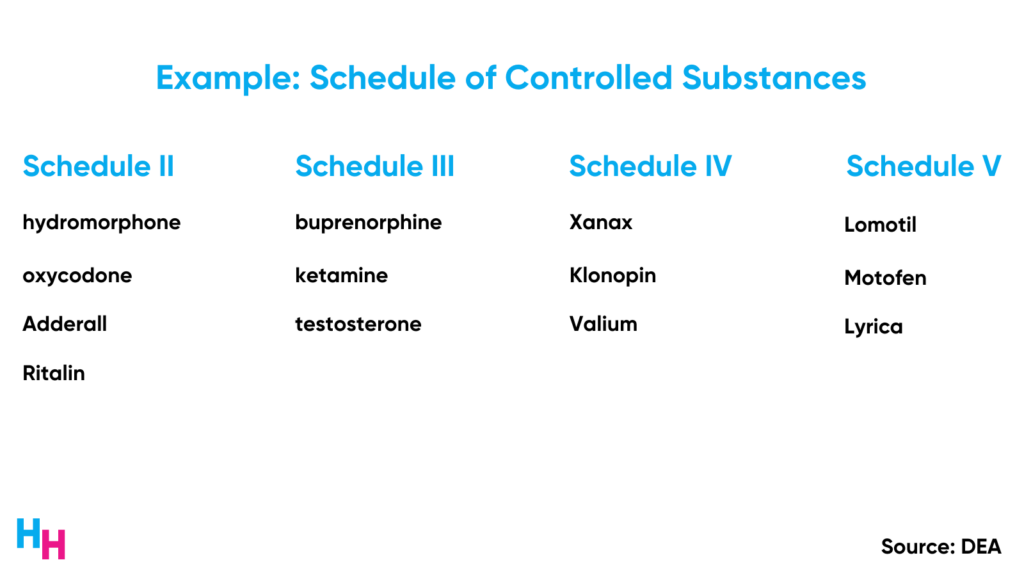
- Providers can only prescribe controlled substances with an initial in-person visit. However, providers may prescribe a max 30-day supply of the controlled medication before seeing the patient in person, known as the “initial prescription period.”
- An initial in-person visit is needed before prescribing Schedule II or Schedule III-V narcotic medications. So, there’s no “initial prescription period.” There’s an exception for buprenorphine when used for opioid use disorder. Buprenorphine will have the initial prescription period, but an in-person visit is still required for the patient to receive more than a 30-day supply.
These new rules aim to prevent overprescribing controlled substances by digital health startups (cough cough… Cerebral and Done).
However, these rules woefully omit any process to ensure continuity of patient care. For example, a patient with opioid use disorder who’s been using virtual digital health company Bicycle Health for treatment would have to end care with them and see a physician in-person only to be reinitiated into medication treatment for opioid use disorder. Initiating treatment is one of the greatest hurdles for someone with substance use disorder. To make them do it twice, after already being an established patient, is sourly misguided.
In response to concerns raised by a record 38,000 comments (see above, for example) on its proposed telehealth rules, the DEA has extended the waiver of the in-person exam requirement by 6 months to address these concerns.
Dash’s Dissection
The DEA’s in-person requirement waiver has significantly impacted patients and digital mental health companies. Despite some bad actors, the overall impact of the waiver has been positive—which is important to understand better the effect of not having the waiver.
Impact on Patients
The clearest patient population to have benefited from the DEA’s waiver is those with opioid use disorder.
Researchers found that among Medicare fee-for-service beneficiaries during the pandemic, medications for opioid use disorder via telehealth was associated with increased medication retention and lower odds of medically treated overdose compared to a similar population pre-pandemic. The researchers also found the percentage of the pandemic cohort receiving opioid use disorder-related telehealth services was 35-fold that of the pre-pandemic cohort. A later study by the same authors found that opioid use disorder-related telehealth was associated with a significantly lower adjusted odds ratio (aOR) for fatal drug overdose than those not receiving opioid use disorder-related telehealth.
Given the evident benefit of opioid use disorder-related telehealth offering medications for opioid use disorder, it’s easy to imagine what will happen if the DEA reverts to pre-pandemic rules requiring in-person visits before receiving medication.
And accessing a new, in-person appointment isn’t easy—especially for a mental healthcare provider. This could take months! Under the DEA’s proposed rule, patients with opioid use disorder on buprenorphine would be out of luck if they can’t find a new provider within 30 days, which is already unlikely.
Aside from increased access to substance use disorder (like opioid use disorder) treatment via telehealth, those with other mental health conditions also benefited from the DEA’s in-person requirement waiver.
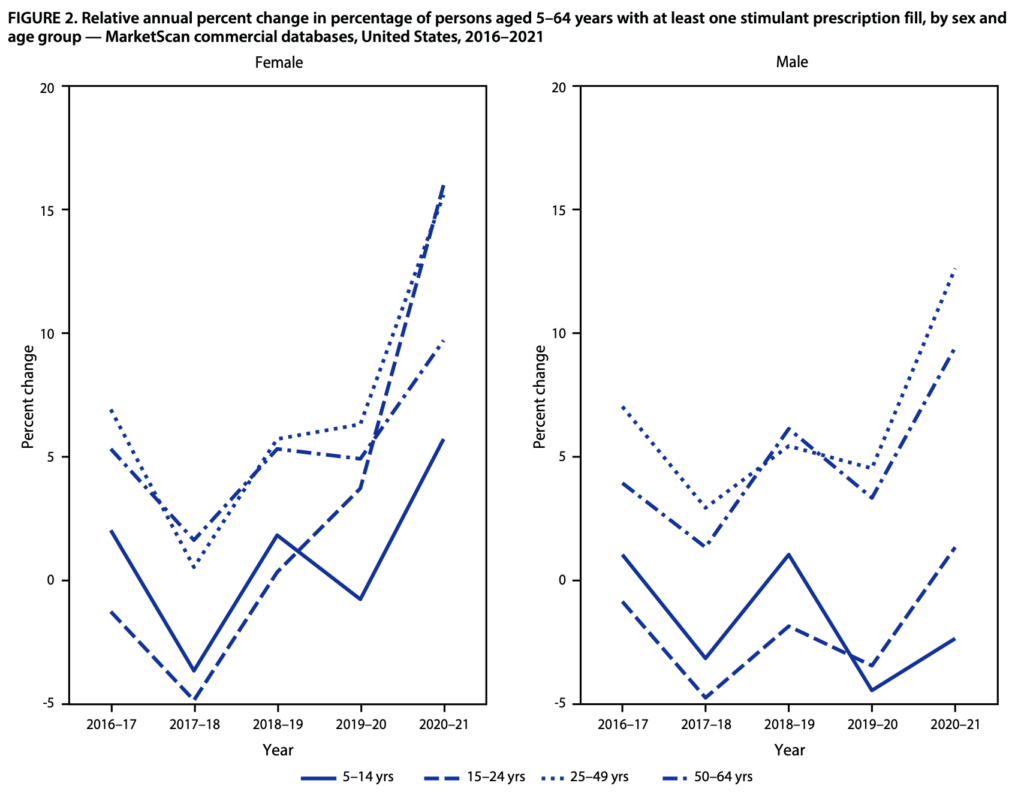
For example, stimulant prescriptions for ADHD (e.g., Adderall—a controlled substance) spiked during the pandemic. The percentage of females 15–44 and 50–54 years with one or more prescription stimulant fills increased between 14.3% to 19.2% during 2020-2021 compared to the period before. For males aged 25–44 and 50–54 years, the percentage with prescription fills increased between 11.1% to 14.7%. Check out the data here.
On the one hand, we can say those who’ve gone years with undiagnosed ADHD finally have access to effective treatment, allowing them to be more productive. On the other hand, we may assume there has been overdiagnosing of ADHD due to poor evaluations. Therefore, some aspect of the DEA’s rule needs to address this overprescribing while balancing the impact of inaccessibility by requiring an in-person visit.
Impact on Digital Mental Health Companies
Digital mental health companies like Ophelia, Boulder Care, and Bicycle Health have helped expand access to substance use disorder treatment, especially during the pandemic. Between Ophelia and Boulder, they account for over one-third of the national net monthly increase in buprenorphine care and are retaining patients for more than 180 days, which is twice the national average.
Despite their great reach and opportunity, Ophelia and Boulder are limiting their patient growth due to uncertainty with DEA rules requiring in-person visits before prescribing controlled substances.
Bicycle Health has already experienced what will likely happen if the DEA removes the waiver. Last year, Alabama started requiring an in-person visit before prescribing a controlled substance. In response, Bicycle Health had to fly out its doctors to Alabama to perform in-person exams for their 500 patients, but 100+ patients didn’t show up. So imagine the impact at scale if/when the DEA reestablishes the in-person requirement.
There are more companies than Ophelia, Boulder, and Bicycle: health and wellness investor Jess Schram wrote about this topic previously and made an excellent market map of companies prescribing controlled substances.

In my opinion, the proposed DEA rules requiring an in-person visit fail to account for health system factors that would inevitably disrupt care for hundreds of thousands of patients needing medication.
Again, “requiring an in-person visit within a month”… when was the last time you were able to schedule a non-urgent new patient appointment in a month?
The health consequences—including overdose—are too extreme for this rule to be established, which is why I’m happy the DEA has extended its implementation for another six months while they figure out what to do.
Lastly, acknowledge that the Ryan Height Act was established in 2008: I was just bar mitzvah’d at the time, and telehealth was almost non-existent! Now, I’m graduating from medical school and entering a field that has fully adopted telehealth and integrated it into workflows. The Ryan Height Act needs an update.
In summary, the DEA’s waiver of the Ryan Haight Act’s in-person exam requirement during the pandemic greatly expanded access to treatment for patients needing controlled substances, especially those with opioid use disorder. As the public health emergency ends, the proposed DEA rules may impact patients and digital mental health companies, potentially disrupting care for many. It’s crucial to consider the implications of these changes and explore ways to maintain the benefits of telehealth while addressing concerns regarding the misuse of controlled substances.
Stay ahead in healthcare with my weekly Healthcare Huddle newsletter, covering digital health, policy, and business trends for 28,000+ professionals. Share with colleagues and subscribe here.