26 June 2022 | Healthcare
Walgreens Moves into Clinical Trials
By workweek
Walgreens is moving into the clinical trial business. They aim to increase patient access and retention in clinical trials by redefining the patient experience.
While the clinical trial arena faces tremendous obstacles in enrolling patients and ensuring they follow up, Walgreens is uniquely positioned to overcome such obstacles given their readily available tools and resources.
The Deets
Walgreens will form partnerships with pharma companies to create patient-centered clinical trial experiences. The plan is to make clinical trial enrollment and future participation as convenient as possible and ensure the trial population is ethnically and racially diverse. Trial participants must be representative of real-world populations since results from clinical trials guide real-world clinical practice. Several patient groups remain underrepresented in trials, which Walgreens wants to address:
- Older adults
- People belonging to racial or ethnic minority groups
- People with comorbid conditions
- Individuals in rural locations
Walgreens can use its clinical data (remember, they own VillageMD) and match its patient population to suitable clinical trials.
Additionally, Walgreens is uniquely positioned to improve travel convenience. Traditionally, patients enrolled in clinical trials must travel to the clinical trial site, which is a major burden and one of the most significant reasons people don’t participate in clinical trials. Think about all of the people in rural areas! Well, around 75% of Americans live within five miles of a Walgreens, which has 9,000 locations in all 50 states.
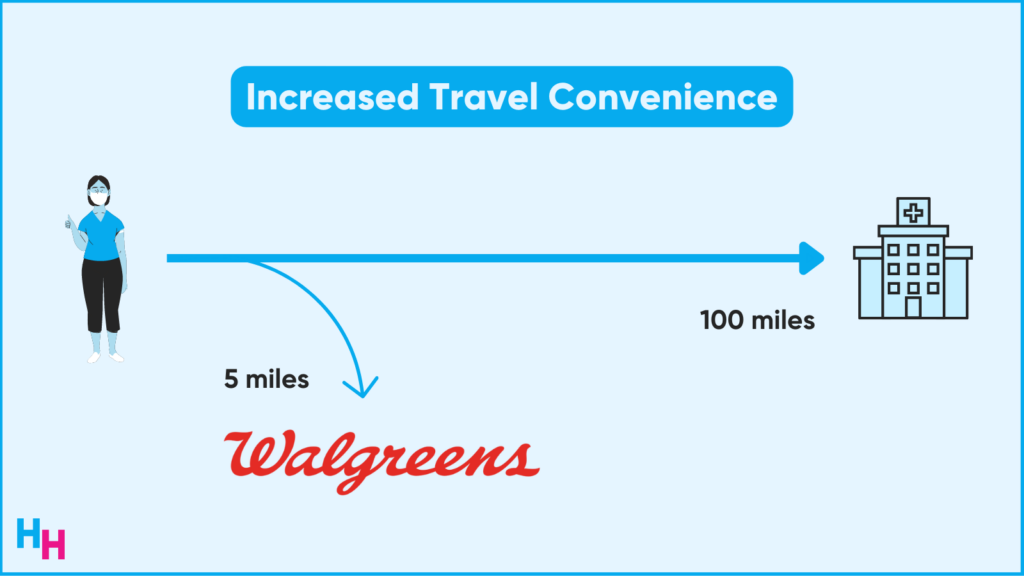
Lastly, Walgreens will take advantage of recent acquisitions to leverage clinical trial convenience: CareCentrix, a home health management company now owned by Walgreens, may be able to run clinical trials in the patient’s home.
My Thoughts
The pandemic has transformed the clinical trial landscape through increased decentralization. Walgreens’ clinical trial initiative is an extension of this “decentralization,” albeit with more flexibility.
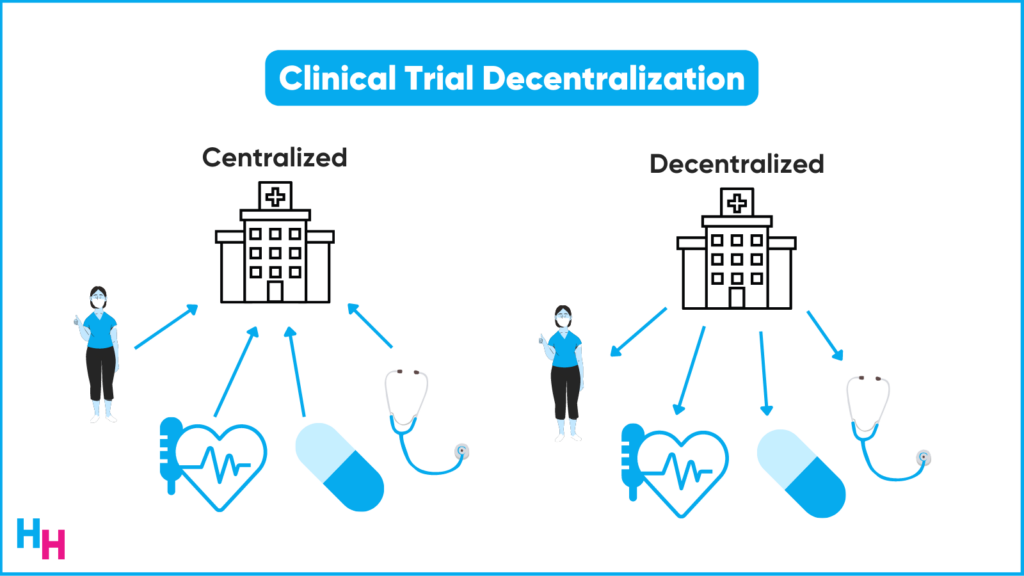
As I mentioned above, the traditional clinical trial required patients to travel to the clinical trial site. It’s not like these sites are just around the corner. According to Deloitte, 70% of potential clinical trial participants in the U.S. live more than two hours from the nearest study center. And, participants may need to travel to the sites frequently.
Since the pandemic prevented in-person visits, decentralization of clinical trials—bringing the trial to the patient instead of the patient to the trial—became very popular. For example, drug administration and assessments in the decentralized clinical trial can be performed remotely.
There’s a continuum of decentralization from complete decentralization to complete centralization. Walgreens falls right in the middle—the “hybrid”.
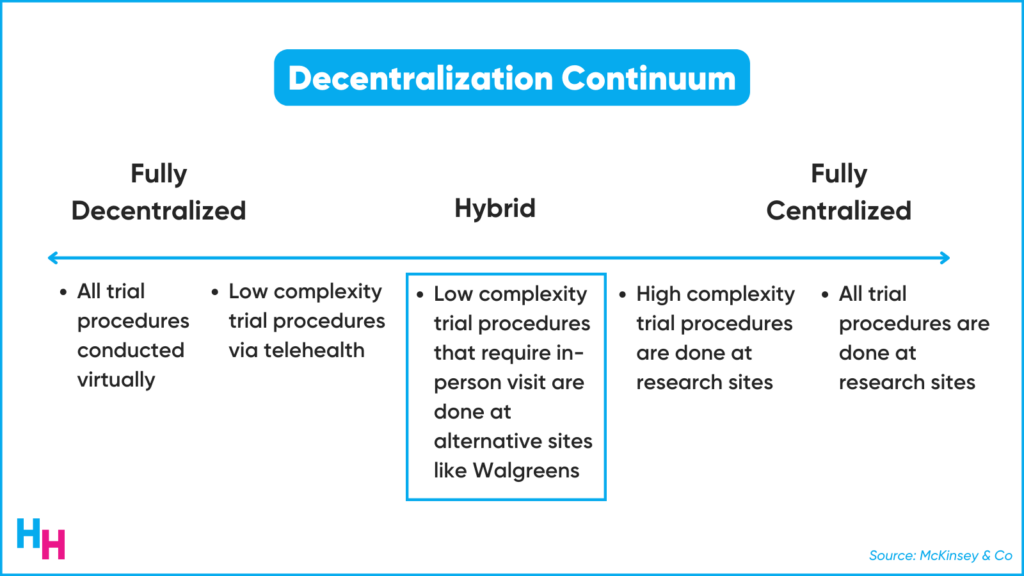
Some aspects of the clinical trial can remain virtual, while others may require participants to come to their nearest Walgreens, which shouldn’t be too far. Overall, the patient experience in the clinical trial is bound to improve given the convenience.
To conclude, the medical system needs more participation in clinical trials, and decentralization is a great way to make that happen. However, there needs to be a significant focus on recruiting diverse populations. Doing so will require increased cultural competency from recruiters of potential participants and radical transparency regarding what the clinical trial is about to build trust.