10 September 2022 | Healthcare
Another Controversial Drug Slated for FDA Approval
By workweek
FDA advisors voted 7-2 in favor of Amylyx Pharmaceutical’s ALS drug known as AMX0035. Meanwhile, these advisors voted 6-4 against the same drug back in March. What accounts for the change in votes and what does this mean for those with ALS?
The Deets
FDA advisors now back Amylyx’s ALS drug, despite voting against it several months ago. Back in March, advisors recommended the FDA hold off on approving the ALS drug until more evidence suggested it works.
The study was a multicenter, randomized, double-blind trial with around 140 participants with ALS. The primary outcome was the rate of decline in the total score on a type of ALS functional scale over 6 months. There was a significant 0.42 points per month difference favoring the drug over the placebo on the functional scale. Despite a modest but significant difference, advisors said the sample size was too small to recommend the drug for FDA approval.
Months later, the turn tables have turned for the following reasons:
- New, compelling stories from patients who were taking the medication.
- Additional analyses from Amylyx.
- A rare, bold move by Dr. Billy Dunn, Director of the FDA’s Office of Neuroscience.
Dr. Dunn made a “promise” with Amylyx. In a sense, he said this to the drugmaker:
If we approve your drug now, would you voluntarily withdraw the drug if your larger clinical trial happening now fails?
The company said yes (not sure if they were smirking or not when they said it), which was enough to win advisors over. The FDA is expected to approve the drug later this month.
Dash’s Dissection
Amylyx’s ALS drug saga reads similar to Biogen’s Alzhiemer’s drug, Aduhelm. The FDA granted Aduhlem accelerated approval despite its shaky efficacy and strong recommendation from an independent review board not to approve it.
This brings us back to the FDA Accelerated Approval Pathway program. This program fast-tracks drugs that meet unfulfilled needs for hard-to-treat diseases like cancer, Alzheimer’s and ALS. These drugs get to the market faster than if they followed the regular approval route. As a result, the accelerated approval program is becoming increasingly popular. Last year, over 20% of the 50 new drugs approved came from this program.
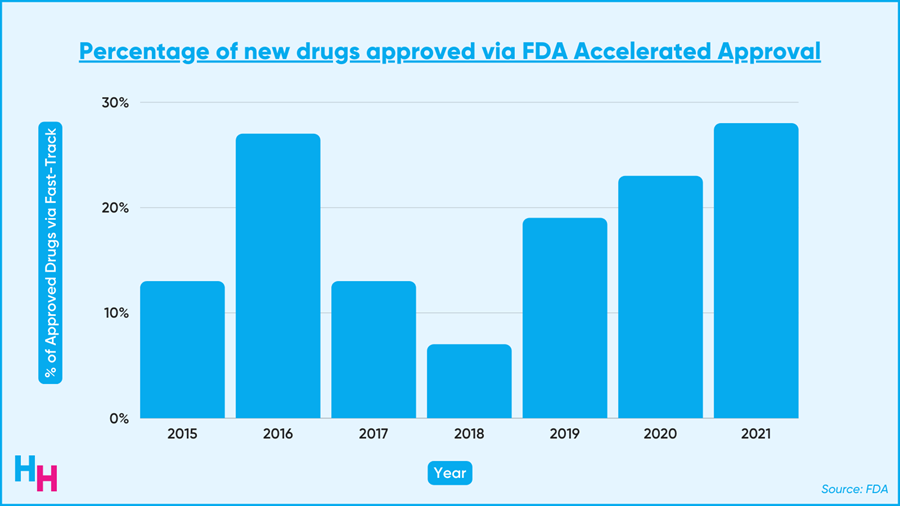
Now, I am extremely skeptical that Amylyx would take their drug off the market if its large clinical trial failed following an accelerated FDA approval. That’s just me, though.
What is different about this ALS drug compared to Biogen’s Alzheimer’s drug is the safety profile. While Alduhelm led to bleeding risks, Amylyx’s ALS drug is considered safe. At the end of the day, if the drug shows even the slightest benefit and is all-around safe, that’s still a bit of hope families fighting ALS can hold on to. From the art of medicine side, even a little bit of hope can help families tremendously.